by Curt Sewell
Summary -- This article describes Carbon-14, and how it's distributed throughout all living objects. We also discuss the basics of its radioactive behavior, and the principles of the Carbon-14 Dating Method, including its assumptions.
In the last part of this article, we discuss some new scientific evidence, showing that some basic assumptions are in error. At least one of these errors actually becomes a strong evidence that the Earth cannot be more than about 100,000 years old.
Setting the Stage
I'd like to begin this article by describing one example of Carbon-14 dating, performed by one of the leading labs in the dating field. Their carefully done report is a classic illustration of the controversy that exists between most secular scientists and most Biblical creationary scientists.
The Hawkesbury Sandstone formation, near Sydney, Australia, is a massive and spectacular mass of hard rock, often used for construction of buildings in Sydney.(1) There are three principle layers of rock -- massive sandstone, sheet sandstone, and some thin mudstone. Although it is massive (7,700 square miles in area and up to 820 feet thick) it shows many of the features of deposition in fast-flowing waters. There are cross-beds, sloping at about 20o, some are up to 20 feet high, within the flat-lying strata. These were probably formed by huge sand-waves, swept by massive water flows. A number of lenses of mudstone contain many fossils, mostly of fish, sharks, and aquatic plants. Geologists have assigned it to the Middle Triassic 'age' (225 - 230 million years old), based on fossil content and the relative sequence of rock layers in the Sydney Basin. This "stratigraphic dating" is the technique most widely used by conventional geologists who believe in the long timescale of the Geologic Column.
The Bundanoon quarry found a finger-size piece of wood impregnated within the hard sandstone. Some Australian creationist scientists obtained part of this wood, and sent it to Geochron Lab in Boston for careful 14C analysis. Contrary to usual practice, they didn't tell the lab where it had been found, or what 'age' they expected it to reveal. This was to prevent possible bias in the dating tests.
The lab applied normal procedures, treating it with hot dilute hydrochloric acid to remove all the carbonates, then with hot dilute caustic soda to remove any humic acids or other organic contaminents. A 13C/12C measurement showed high probability that modern contamination was not a factor. The sample wood was found to contain measureable 14C, and the final age was determined to be 33,720 +/- 430 years BP.
That "age" is obviously very much younger than most secular scientists could accept, and very much older than most creationists think it really should be. We'll use that "age" as an example in the main discussion about Carbon-14 dating, and the probable sources of the controversy. That comes in the last several pages of this article.
Introduction to Carbon-14 Dating
One of the elements necessary for life is carbon. All plants and animals contain a considerable amount of carbon, in addition to other materials. The atmosphere contains a lot of carbon dioxide (CO2). Plants "breathe in" CO2 , and photosynthesis in the plant splits off the oxygen, and keeps the carbon for use as food. Excess oxygen is just "exhaled" back into the atmosphere. Animals and humans eat plants, and retain a large amount of their carbon. They also breathe in oxygen and breathe out CO2. Thus there is a constant exchange of carbon between the air, plants, and animals (including humans). Biologists refer to this as the "carbon cycle of life."
There are also inanimate objects, such as limestone and some other kinds of rocks, coal, oil, natural gas, etc., that contain carbon. But these are usually considered to be too ancient to be dated by the 14C method. This opinion becomes the important "punch-line" in the last part of this article.
When discussing specific isotopes, nuclear physicists use leading superscripts to denote atomic weight. For example, carbon exists as three kinds of isotopes -- 12C, 13C, and 14C. These have identical chemical properties, and they each contain 6 protons in their nucleus. The only difference (almost) is the number of neutrons in the nucleus, and thus their atomic weight. Also 14C is mildly radioactive, randomly emitting weak beta particles (nuclear electrons), and having a half-life of about 5,730 years. The atom left behind after that emission is normal nitrogen, indistinguishable from all the 14N in the atmosphere.
![]()
How Does Carbon-14 Dating Work?
When a plant or animal (or human) is alive, it contains the same fraction of 14C/CTotal as does the atmosphere around it. But when it dies, its fraction of 14C begins to diminish (decay), since 14C is radioactive. So when an ancient sample is analyzed, the relative amount of 14C that's left is a measure of how long that sample has been dead. As a practical limit, samples longer than several half-lives (a few tens of thousands of years) cannot be precisely dated, because so much of the 14C has decayed.
Notice that I use the term 14C/CTotal to mean the fraction of 14C compared to the total of all three isotopes of modern carbon. Natural carbon is almost completely 12C. The fraction of modern 14C is only about 1.176 x 10-12.
Where does Carbon-14 Come From?
It is continually being generated in the upper atmosphere. As cosmic rays (highly ionizing radiation from outer space) enter the atmosphere, some collide with atoms, knocking off neutrons. When one of these neutrons is absorbed by an atom of nitrogen (14N), that atom emits a proton, changing that atom to 14C.
![]()
Most of these new atoms of 14C combine with oxygen to form carbon-dioxide (CO2) which is mildly radioactive. Wind and other sources of diffusion distribute these new molecules throughout the Earth's atmosphere. These eventually approach equilibrium with other CO2 atoms and mix into the "carbon cycle of life."
Some Disturbing Assumptions
Willard F. Libby invented this dating system, and wrote the definitive book on the subject. Among other puzzling factors are the Specific Decay Rate (SDR) and the Specific Production Rate (SPR). If the atmosphere is in 14C equilibrium, these two numbers should be the same. But they're not. The SPR of 14C was thought to be 18.8 atoms per gram of total carbon per minute. But the SDR was only 16.1 disintegrations per gram per minute -- as if there hadn't been enough time to reach equilibrium! This troubled Libby, since he believed the world was many millions of years old. He wrote: (2)
"If one were to imagine that the cosmic radiation had been turned off until a short while ago, the enormous amount of radiocarbon necessary to the equilibrium state would not have been manufactured and the specific radioactivity (SDR) of living matter would be much less than the rate of production (SPR) calculated from the neutron intensity."
Since creationary scientists believe in a relatively recent creation, they said that Libby was unknowingly agreeing with the Bible's account of a recent creation. But Libby chose to ignore this discrepancy and attribute it to experimental error. Libby thought the half-life of 14C was 5,568 years. Early measurements used this number.
Basic Presuppositions
There are a number of presuppositions, some of which are good, while others may not be. These include:
1) While a plant or animal is alive, it contains the same relative fraction of 14C/CTotal as does the surrounding atmosphere. (Probably true.)
2) At the time of death, no more exchange of carbon occurs, and thus the fraction of 14C/CTotal will diminish as the 14C decays. (Probably true.)
3) The decay rate of 14C has always been a constant. (This is widely believed to be true, although evidence is growing that it may not be.)
4) By careful construction of equipment, the fraction of 14C/CTotal in a sample can be determined. (Probably true, although subject to experimental error.)
5) The atmospheric fraction of 14C/CTotal has always been a constant, the same as can be measured at the present time. (This is almost certainly false! It's impossible to measure today what it was like in prehistoric times.)
Measurement Methods
For the first 25 years or so, the 14C in the sample was measured by counting the beta radiation, by either internal gas proportional counters or by scintillation counters. However these are both inefficient because of background radiation from cosmic rays and nearby extraneous radiation. Heavy shielding and anti-coincidence counters were used to reduce those effects. But these methods each required large quantities of the sample material.
In the mid-1980's a new method, Accelerator Mass Spectrometry (AMS) was developed. Now relatively tiny bits of the sample become usable, and background is minimized.
A Huge Problem
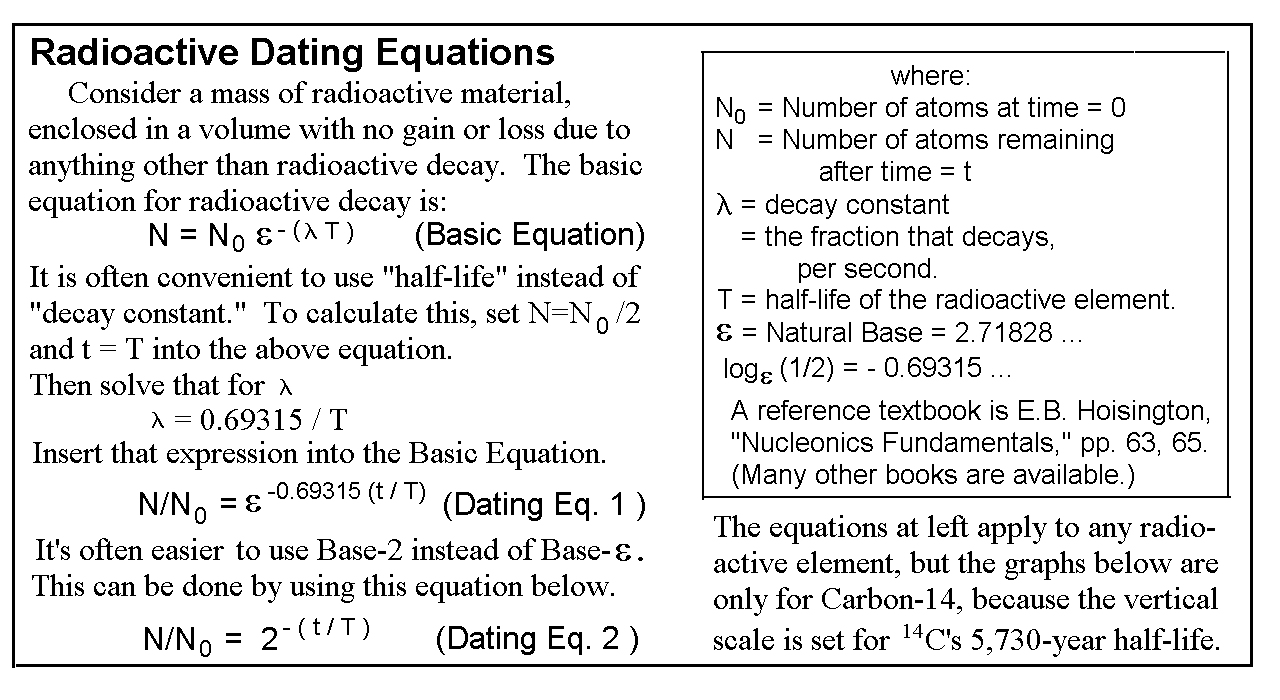
AMS mchines can very accurately measure the ratio of isotopes within the sample to be age-dated. But in order to determine the accurate age of the sample, they must confirm the validity of the five presuppositions listed above. And No. 5 is impossible to experimentally confirm or reject -- they can't go back in time thousands of years and measure the isotopes in the atmosphere at the time of death or burial of the sample! So they must rely on their ideology of uniformitarianism, "the present is the key to the past." They say, "We'll have to assume that today's atmospheric content is the same as what it has always been, down through prehistoric times."
Since they can't obtain the fraction N/N0, as the derivation of the Dating Equations require, they substitute the fraction N/NModern instead -- (look at the box "Radioactive Dating Equations," on the next page) They've defined a new term, "pcm," meaning "percent of modern carbon," that refers to the fraction N/NModern. That is really based on their belief that the atmosphere has always had the same ratio of 14C/12C as it has today. They've chosen to ignore the possibility of an ancient environmental catastrophe that may have had a long term effect on the atmospheric makeup. (Incidentally, modern standards are based on pre-World War II quantities, so as to avoid the 14C that's caused by atomic explosions. And that is reasonable.)
 However
there are many legends in the folk-lore of civilizations around
the world, describing how such catastrophes have occurred. Most
important of these, the Bible has graphic descriptions of the
Great Flood of Noah, which covered the complete land surface,
killed almost all air-breathing animals, and lasted for a full
year. Imagine the world-wide devastation that must have occurred
in the runoff of all that water. We saw a tiny example in 1980,
when Mount St. Helens erupted. Millions of trees were uplifted
and buried. If something like that was world-wide, that would
certainly have disrupted the entire carbon cycle of life, and
must have had a drastic effect on atmospheric content.
However
there are many legends in the folk-lore of civilizations around
the world, describing how such catastrophes have occurred. Most
important of these, the Bible has graphic descriptions of the
Great Flood of Noah, which covered the complete land surface,
killed almost all air-breathing animals, and lasted for a full
year. Imagine the world-wide devastation that must have occurred
in the runoff of all that water. We saw a tiny example in 1980,
when Mount St. Helens erupted. Millions of trees were uplifted
and buried. If something like that was world-wide, that would
certainly have disrupted the entire carbon cycle of life, and
must have had a drastic effect on atmospheric content.
Some Evidences that Trouble Uniformitarians
The majority of items that are usually dated by the 14C method are archaeological or historical artifacts, and sometimes things that relate to studies of ancient populations. These are items that are first estimated to be younger than a few tens of thousands of years old.
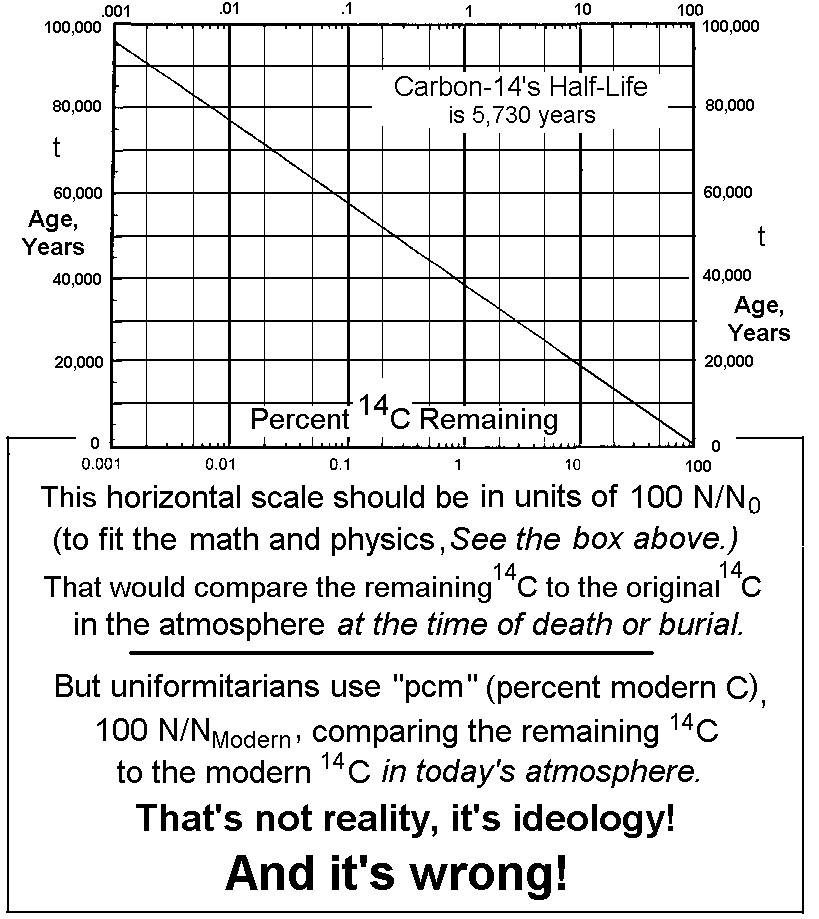
The fraction of 14C remaining after 5,730 years is 1/2, and it drops another 1/2 during the next 5,730 years. By the time 57,300 years (10 half-lives) have gone by, there's only about 0.1 % of the original 14C remaining, and after 20 half-lives it has decreased by 220 (to about 1/1,000,000). This would be entirely too small a quantity to measure, using any method. Most of today's scientists believe that fossil fuels (coal, oil, etc.) and some kinds of rocks (limestone, etc.) are many millions of years old. Thus they don't think they could be carbon-dated, and therefore they don't usually try.
In the older beta-counting method, the very oldest samples that could be measured with any degree of accuracy were some 30 thousand years of age. When the Accelerator Mass Spectrometer (AMS) method was developed, its increased sensitivity was so much better that chronologists expected to be able to measure samples approaching 100,000 years in age.
But a problem developed. Every test that was made, trying to calibrate their equipment with zero 14C present, failed. Things like natural gas, coal, and even inorganic items like various carbonate rocks contained enough 14C to be dated at between 30,000 and 60,000 years of age. Even diamonds contained measureable 14C. These were all thought (according to the evolutionist geologic scale) to be millions of years old, far too ancient to still contain any carbon-14 at all.
Chronologists began a major effort to improve their equipment and their techniques. Many papers have been published, in professional peer-reviewed journals, speculating about what caused this disappointing result. But no satisfactory explanation has been forthcoming. All of the tests seemed to show that the 14C in those very ancient samples was actually part of the sample! Yet if they admitted that such a sample contained 14C, that would violate their entire uniformitarian belief system. So they began to refer to it as "contamination" or "background," even though those words didn't really fit the case.
But the one thing they wouldn't acknowledge was their assumption about the geologic time scale -- that those samples may not have been millions of years old. That assumption is part of the scientists' belief in a purely materialistic origin of the earth and its contents. They refuse to accept the Biblical account. Materialism has grown into a secular religion for the secular scientific community!
But How Can This Be?
Then creationary scientists began a careful study of this problem. The Institute for Creation Research (ICR) sponsored a project they called Radioactivity and the Age of the Earth (RATE), and it's beginning to show some solid evidences that support the Biblical time scale -- support with scientific evidences, not just religious arguments. One paper by Dr. John Baumgardner et al.,(3) documents about 70 articles that have been published in professional journals during the last 20 years, discussing this problem. These showed that many samples from various eras of the geologic column, that should have been 14C-dead, actually contained as much as 0.5 % of the modern value of 14C. RATE even purchased samples of coal from the U.S. Department of Energy Coal Sample Bank at Pennsylvania State University. When these were "dated" by several qualified AMS laboratories, they all showed amounts of 14C ranging from 0.1% to 0.5% of the amount in our modern atmosphere. They even measured similar results from diamonds -- some of the oldest items on earth! According to the above graph, (evolutionists say that) their age should be between 40,000 and 60,000 years old. But we showed that the uniformiatarian's use of "pcm" (comparison of the 14C in the ancient sample, to the carbon in the modern atmosphere of Earth) is like comparing apples to oranges. It has little relation to reality.
That age is many times younger than the millions of years that uniformitarians believe, but it's still much older than is shown by a literal interpretation of the Bible. Read on -- we'll show a surprising correspondence between the actual ratios and the Biblical time frame.
Dr. Baumgardner's explanation involves the tremendous devastation that came upon the Earth at the time of the Great Flood of Noah. Prior to that time, the Earth was a paradise, containing lush vegetation and many sorts of animal life. He cited estimates showing that the carbon in the biomass was probably up to 100 times as much as it is today. (4)
But when the Flood came, the majority of all that carboniferous material was buried -- gone from the "carbon cycle of life." That would obviously have a drastic effect on the atmosphere.
The Secular Viewpoint
Let's try and compare two different ways of calculating 14C dates, or at least calculating a reasonable explanation showing that the opposite view was unreasonable, and why. Then we'll apply them each to the fossil wood we described at the beginning of this article. That story told how Geochron Lab. prepared the sample -- they cleaned it first with hot dilute hydrochloric acid, then with hot dilute caustic soda, then converted it to CO2 gas, and put it through an Accelerator Mass Spectrometer (AMS) to find the relative amounts of carbon isotopes. Their report said that the sample was 33,720 years BP (before present). From that age, we can calculate (using Dating Equation 2) that they must have found the "pcm" to be 1.69%. It's customary for secular labs to use the "pcm" (N/NModern) ratio to arrive at an age (even though the derivation shows that it should have been N/N0 -- see the sentences just below the Graph shown on a previous page). Remember that N is the amount of 14C found today, and N0 is the amount of 14C that had been in the original. fossil. They've relied on their belief-system to try and find the value of N0 (the amount of 14C present in the sample when it was first buried), and we will show that in this case they reached a totally wrong date of 33,720 years BP.
Ancient Rich Climate
Scientists on both sides of the Creation / Evolution debate all agree that there was a time in the ancient past when the earth was warm, moist, and rich in vegetation. The fossil record furnishes much evidence demonstrating that both vegetation and animal life were huge and dense. Secular geologists call this the Carboniferous Period, occupying from 345 to 280 million years ago. This is part of the Geologic Column, the framework of the uniformitarian time scale -- one of the most sacred doctrines of materialistic science.
On the other hand, creationary scientists reject the "Geologic Column" time scale, and say that this lush vegetation and prolific animal life must have been just before the Great Flood of Noah, between 4 and 6 thousand years ago. Both sides agree that this period furnished the material for most of the fossil fuels -- coal, oil, and natural gas (even though they disagree drastically on the age of that luxuriant period).
The Creationist Viewpoint
Now let's look at the creationist picture. They would have used about the same initial steps -- cleaning the sample to eliminate contamination, converting it to CO2 gas, and measuring the carbon isotopes in an AMS machine. The difference between the two methods lies in the interpretation. A creationist would have recognized that, with that small amount of 14C present, that sample must have been thousands of years old. So they would have tried to compensate for the different climate in ancient times. They might guess that it may have been buried in the Flood. How would that have affected the carbon isotope ratios?
For one thing, the biosphere before the Flood must have held very much more carbon (mostly 12C) than is there now -- as we pointed out above, possibly 100 times as much as today's. At the same time, the amount of 14C was probably less than today's. Both of these would have lessened the original 14C/12C ratio in the fossil.
The Bible says that the Flood was world-wide, and destroyed all of the Earth's surface. Even the mountaintops were covered. The runoff of that much water must have gone on for quite some time as the present ocean basins sank, some land areas uplifted, high mountains were formed, and tectonic movements took place. Huge volumes of water ran off of the land surface. The bulk of the forests, and also the drowned carcasses of animals, were swept into piles and buried. Many "dinosaur graveyards" have been found, tumbled into huge piles of bones. This removed a large fraction of the carbon from the biosphere. Carbon that had been contained in trees and animals, and available for exchange in the "carbon cycle of life" was now covered by hundreds or thousands of feet of sedimentary rocks.
What about the amount of 14C that had been available to that life cycle? A lot of that was also buried. Remember that 14C is generated by cosmic rays, in the upper atmosphere. Before the Flood, there was probably a lot of water vapor (or a vapor canopy) surrounding the earth. There is also good evidence that the earth's magnetic field was much stronger in pre-Flood times than it is today. Both of these effects would have shielded the earth from cosmic rays, so that there were not nearly as many as bombard us today, and therefore the amount of 14C generated each hour was probably much less than today.
According to the Bible, there were about 1650 years between Creation and the Flood, and the Creation was about 6000 years ago. If 14C production began at the time of Creation, and has held a steady rate since then, we could guess that just before the Flood there may have been about 1650 / 6000 (or 28%) as much as in today's level. That's a fairly good starting point for guesswork.
The box on the next page has four examples of calculations, just to give the reader a "feeling" of how variations in the ancient atmosphere may affect the age-dating by today's scientists. In all four, I've assumed that a piece of wood was buried during the Flood, 4,350 years ago. I've assumed two numbers for the 12CO2 in the atmosphere at the time of burial, and two numbers for the 14CO2 amount at that time. I've shown how those bits of wood would be measured in an AMS machine today, and what age would be assigned. They would show ages ranging from 33,500 years BP to 61,500 years BP. Remember that the Australian wood buried in sandstone was dated at 33,720 years BP. So this is an excellent example to account for why 'ages' much more ancient than 4,350 years arise when the Flood is not taken into account.
Conclusions
First, the finding that
measurable and reproducible amounts of 14C in fossil material
such as coal and other materials previously thought to be very
ancient is a powerful refutation of the geological timescale of
millions of years. This should force a complete re-examination
of the entire scientific system of dating.
Second, we've shown the necessity of recognizing the importance
of atmospheric carbon content at the time of death or burial of
the sample under test. The concept of uniformitarianism -- that
"the present is the key to the past" -- is simply not
true. The ancient atmosphere was different from today's, and
that has a strong effect on determining the date of the sample
under test.
Third, the Creation-vs-Evolution conflict is not a contest
between science and religion. Each side uses scientific methods,
but also both sides rely on faith. One has faith in God and His
Bible, the other has faith in materialistic naturalism. One system
is theistic, the other is atheistic.
|
How the Relative Composition of the Ancient Atmosphere Affects the Dating |
||||
|
We'll look at four hypothetical cases of wood caught up in a massive flow of water, which also carried a heavy load of sand, so that when the water drained off, the pieces of wood were buried in sand, and the whole mass later hardened into a huge mound of sandstone. These four cases differ only in the assumption of different amounts of 12CO2 and 14CO2 , compared to those in today's atmosphere. In each case, we assume that the Flood was actually 4,350 years ago. |
||||
| Case 1 | Case 2 | Case 3 | Case 4 | |
| Pre-Flood 12CO2 = | 10 times today's | 10 times today's | 100 times today's | 100 times today's |
| Pre-Flood 14CO2 = | 0.3 times today's | 0.1 times today's | 0.3 times today's | 0.1 times today's |
| True Age, years = | 4,350 | 4,350 | 4,350 | 4,350 |
| Amount of 14C absorbed into piece of wood, compared to similar wood in today's atmosphere. | 0.3 / 10 = 0.03 | 0.1 / 10 = 0.01 | 0.3 / 100 = 0.003 | 0.1 / 100 = 0.001 |
| In each case, the amount of 14C is reduced by 0.59, during the 4,350 years of radioactive decay. That leaves this amount of 14C for the AMS machine to accurately measure. | 0.03 x 0.59 = 0.0177 | 0.01 x 0.59 = 0.0059 | 0.003 x 0.59 = 0.00177 | 0.001 x .059 = 0.00059 |
| They call this "pcm." | 1.77 pcm | 0.59 pcm | 0.177 pcm | 0.059 pcm |
| Here's their "Dated Age (BP)" | ~33,500 BP | ~42,500 BP | ~52,500 BP | ~61,500 BP |
| After measurement, the operators calculate the age, using the measured isotope ratios, and assume that the ancient atmosphere was just like today's. (pcm) | Remember that in all four cases we began with the assumption (Years Before Present) of an actual age of 4,350 years! The only difference in the four is the assumptions about the ancient atmospheric makeup. | |||
Footnotes:
(1) This opening account is abstracted from the article "Dating Dilemma," by Andrew Snelling, in the June-August 1999 issue of "Creation ex nihilo," pp.39-41.
(2) Libby, W.F., Radioactive Dating, (Chicago: University of Chicago Press, 1952, 1955), page 7.
(3) "Measureable 14C in fossilized organic materials: Confirming the young earth creation/Flood model," by John R. Baumgardner, D. Russell Humphreys, Andrew A. Snelling, and Steven A. Austin, a paper given at the 2003 Fifth International Conference on Creationism. It was also posted at the American Geophysical Union Fall Conference in San Francisco on December 8-12, 2003. This can be found on the Internet at http://www.icr.org/research/icc03/pdf/RATE_ICC_Baumgardner.pdf
(4) Brown, R.H., "The Interpretation of C-14 Dates," Origins, 6(1979), pp. 30-44.
Postscript to Carbon-14 Shows the Earth is Young
I received an attempt at rebuttal from an anonymous evolutionist friend of an acquaintance. He tried to show that if a buried mass of carboniferous material (such as coal) is exposed to a source of slow neutrons, then Carbon-14 can be formed by neutron capture by C-13. To a certain extent he may be correct, but he misses the mark by many magnitudes. Those who are interested in debunking claims of excess C-14 in ancient artifacts should consider Dr. Paul Giem's article "Carbon-14 Content of Fossil Carbon," located at http://www.grisda.org/origins/51006.htm. He discusses the problem of excess C-14 with considerable detail, and lists 77 published reports of excess C-14. He then discusses several explanations that have been used in attempts to "debunk" claims by short-age proponents (including C-13 capture of neutrons). He shows that these are all very inadequate. The evidence still shows that a life-containing Earth must be less than about 100,000 years in age. --Curt Sewell, June 2004
Posted 5/6/04